- Valence Electrons Definition
- Valence Electrons In Alcl3
- Valence Electrons In Oxygen
- Valence Electrons Periodic Table
- 1 Valence Electron Group
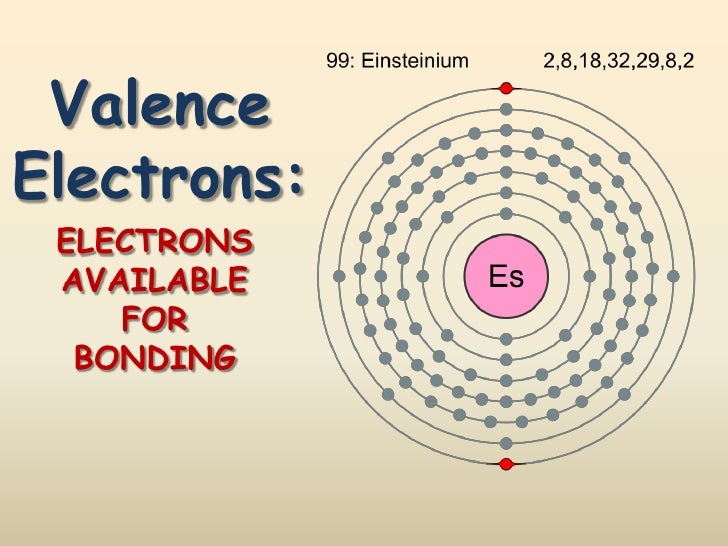
The total valence electrons of the nitrogen and four hydrogens is 9 electrons. Since there is a positive charge of 1+ that means there is one less electron, so there will be a total of 8, which are represented by the four bonds as lines. Affinity publisher demo. Valence electrons are the s and p electrons in the outermost shell. The electrons present in the inner shell are core electrons. When we study and observe the atom of an element, we come across tiny subatomic particles called valence electrons. Lewis structures help us to track the valence electrons and predict the types of bond. Zirconium has four valance electrons, with two in the 4d level and two in the 5s level. This allows it to combine with other elements and ions in different configurations. It has valence charges of +2, +3 and +4. Office 2011 keys for mac.
 Also found in: Thesaurus, Medical, Acronyms, Encyclopedia, Wikipedia.
Also found in: Thesaurus, Medical, Acronyms, Encyclopedia, Wikipedia. Valence Electrons Definition
valence electron
 n.
n.va′lence elec`tron
n.
Valence Electrons In Alcl3
| Noun | 1. | valence electron - an electron in the outer shell of an atom which can combine with other atoms to form molecules electron, negatron - an elementary particle with negative charge |
Valence Electrons In Oxygen
Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.
Valence Electrons Periodic Table
Link to this page:
1 Valence Electron Group





